![]()
![]()
K.E. of Photoelectron
Was
Determined By:
| Flash |
The white light was unable to discharge the electroscope (eject the electrons) but the ultraviolet light could. Why???
The ultraviolet light had a higher frequency ....
... a higher energy.
b) nature of emitting metal
Typical Plot of Frequency of
Light vs. K.E. of
Photoelectron
| KE of Photoelectron |
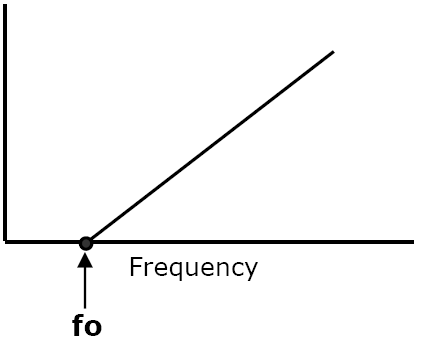 |
fo - Threshold Frequency - frequency below which no photoelectrons will be emitted
Below Threshold?
No Emission
Above Threshold?
Emission
![]()
Summary of
 Photoelectric Effect
Photoelectric Effect
![]()

Enrichment
©Tony Mangiacapre., - All Rights Reserved
[Home]
Established 1995
Use any material on this site (w/ attribution)