![]()
![]()
Man tossing hot and cold water balloons
(thermal infrared!)
School Blocks YouTube? Click on video link below
B) Einstein explains photoelectric effect
1) Light intensity and electron emission explained.
| Photons are received individually | |
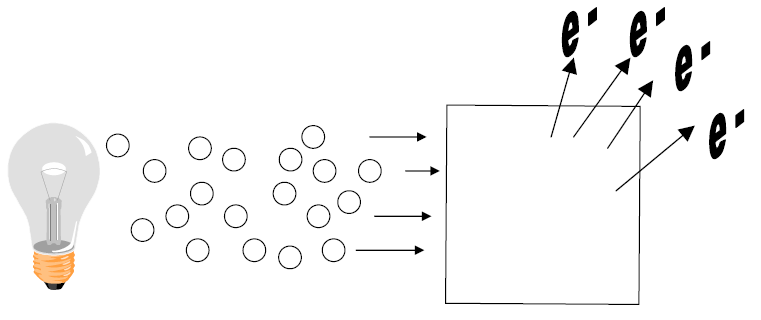 |
|
| Each photon emits e - | |
|
|
|
|
Light emitted as particles
|
|
|
Bright Light
|
Dim Light |
|
|
|
|
(Lots of photons) |
(few photons) |
|
High Frequency Light |
Low Frequency Light |
|
|
|
|
(High Energy Photons) |
(Low Energy Photons) |
Brightness (Intensity) - measure of # of photons emitted.
Frequency measure of
energy of light
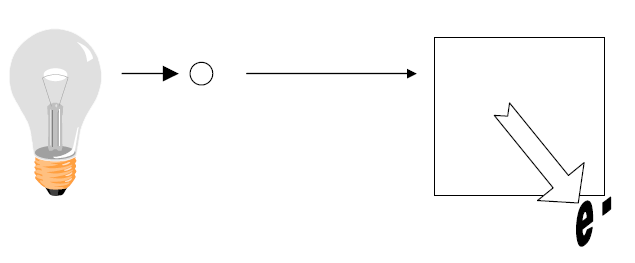 |
|
|
Photon Energy = 5 eV |
KE = 2 eV |
**eV - electron volts - unit of energy
How much work did this photon have to do to remove the electron?
Answer: 3 eV
What would happen if a photon
of 2 eV hit the same metal?
 |
|
| Photon Energy = 5 eV | KE = 2 eV |
Photon would not have enough energy to emit an electron
 |
|
| Photon Energy = 5 eV | KE = 2 eV |
![]()
![]()
ŠTony Mangiacapre., - All Rights Reserved
[Home]
Established 1995
Use any material on this site (w/ attribution)