![]()
![]()
NOVA scienceNOW | Amazing Atoms | PBS
"Here is the latest, atomically correct, version of our old friend, the atom."
School blocks YouTube? Use file below.
NOVA scienceNOW Amazing Atoms PBS.mp4
showmethephysics.com
Ex) How much energy is absorbed when an electron in hydrogen moves from the energy levels n = 2 to n = 5?
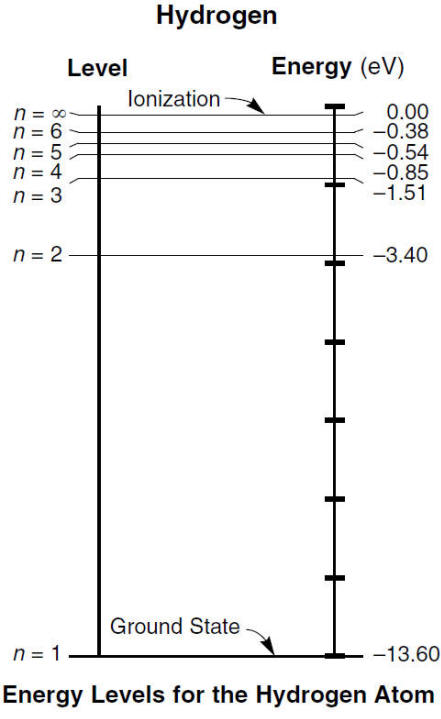
Subtract the absolute values to find the energy needed to be absorbed
Answer
3.4 eV - .54 eV = 2.86 eV
Ex) How much energy is needed for an electron in the n = 1 energy level to move to the n = 2 energy level?
n = 1 -13.6 eV
n = 2 -3.40 eV
Throw out the
negative signs and subtract
= 13.6 eV - 3.4 eV
= 10.2 eV Absorbed
Frequency of photon absorbed
E = hf
h = 6.63 x 10-34 Js
E = 10.2 eV
Convert eV to Joules
page 1 Reference
1 eV = 1.6 x 10-19 J
E = 10.2 eV x 1.6 x 10-19 J
= 1.6 x 10-18 Joules
E = hf
1.6 x 10-18 Joules =
(6.63 x 10-34 Js) f
f = 2.4 x 1015 Hz
Ex) Which energy level transition in MERCURY will produce a photon emission of .22 ev?
c to b transition
(Difference in energy
between b and c is .22 eV)
5) Ionization Energy - minimum energy needed to remove an electron from an atom.
Ionization Energy - greater than or equal to energy at that level.

Ionization Energy of Hydrogen
n = 1
13.6 eV or greater
n = 2
3.4 eV or greater
n = 3
1.51 eV or greater

Ex) A photon having an energy of 15.5 electron volts is incident upon a hydrogen atom in the ground state.
The photon may be absorbed by the atom and
a) ionize the atom
b) excite the atom to n = 3
c) excite the atom to n = 2
d) excite the atom to n = 4

a) ionize the atom
![]()
 Electrons
Energies?
Electrons
Energies?
![]()
ŠTony Mangiacapre., - All Rights Reserved
[Home]
Established 1995
Use any
material on this site (w/ attribution)