B) Bohr Model of Atom
Early 1900's - Danish scientist Neils Bohr discovers
the ____________________.
__________________ in atoms are ____________________________
Hypothetical Element X
Excited Electron - Energy of ______ eV
Excited Electron - Energy of ________ eV
Ground State - Energy of ________
When electron falls back to ground state, what energies is it capable of
giving off?
1 eV = 1.6 x 10-19 J p. 1 Reference
Each energy transition ________________________
Background
Electrons give off ______________ when they return to the ground state after being
________________ by an energy source
Bohr studies the light given off by a '_____________________'
'Black Body' = heated body that gives off ______________________________
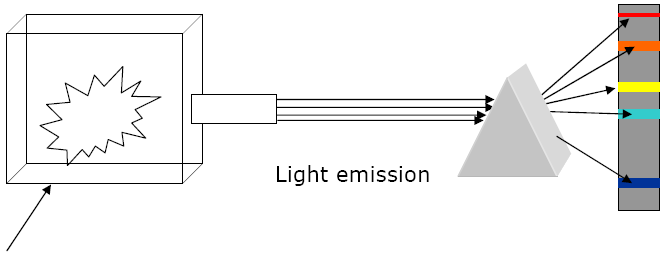
Material was heated and the _
______________________
Every Energy level transition ______________________________
How many different
spectral lines can be produced
by an atom with 5 energy levels?
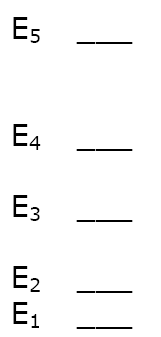
Answer
Black Body Radiation Results:
1. Only the high _______________________________
| R | O | Y |
G |
B |
V |
|
|
|||||
|
|
|
||||
|
|
|||||
2. Electrons in atoms are _______________________________
3. Each element gives off ______________________________
Answer
1. Spectral lines used to: